Abstract
Introduction: Renal light chain (AL) amyloidosis typically manifests as proteinuria with or without renal failure and is associated with a risk of progression to renal replacement therapy (RRT). A significant reduction in circulating amyloidogenic light chain is needed to achieve a renal response. Current renal response criteria are binary defining a renal response as >30% reduction in 24-h proteinuria without worsening estimated glomerular filtration rate (eGFR). Several studies suggest that greater reduction in proteinuria following successful therapy improves renal and overall survival.
Methods: AL amyloidosis patients diagnosed between 2010 to 2015, achieving at least hematological partial response (hemPR) to therapy and with renal involvement (defined as 24-h non-selective proteinuria >0.5 g/24-h) were included. Four predefined renal response categories were formulated based on reduction level in pretreatment 24-h proteinuria in the absence of renal progression (≥25% decrease in eGFR): renal complete response (renCR, 24-h proteinuria ≤200 mg/24-h); renal very good partial response (renVGPR, >60% reduction in 24-h proteinuria); renal partial response (renPR, 31-60% reduction in 24-proteinuria); and renal no response (renNR, 30% or less reduction). Renal response was assessed at landmark (6-, 12-, and 24 months from treatment initiation) and as best renal response. Graded renal responses were assessed as predictors for time from diagnosis to RRT and overall survival.
Results: Seven hundred and thirty-seven patients were included. The median age was 63. The breakdown of renal stage was: I, 34%; II, 52%; and III, 14%. Eighty percent of patients received 1 line of therapy within 12 months of their diagnosis. Bortezomib-based therapy was given to 60% of the patients; 28% received autologous stem cell transplantation (ASCT) as their first line therapy. Hematological CR was achieved in 44% of patients, followed by hematological very good partial response (38%) and hemPR (18%). RRT was required during follow-up in 15% of patients (n=108) with a median time from diagnosis to RRT of 18 (IQR 6-43) months. Twenty-eight percent of the patients died. The median follow-up of the surviving patients was 69 months (IQR 56-86).
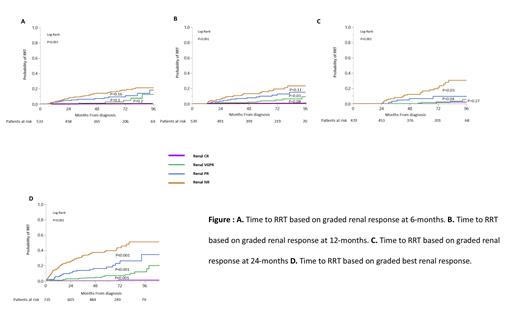
Reduction in 24-h proteinuria from baseline improved over time with a median reduction of 34%, 50% and 71% at 6-month, 12-month, and 24-months, respectively. At best response, renCR, renVGPR, renPR and renNR were achieved in 27% (n=199), 34% (n=247), 15% (n=112) and 24% (n=179) of patients, respectively. The median time to best renal response among renal responders was 17 (IQR 8-31) months, longer for renCR (23 months, IQR 10-40) compared to renVGPR (16 months, IQR 9-27) or renPR (11 months, IQR 6-19). A renal response as early as 6 months after therapy initiation was able to predict time to RRT with an increase in RRT risk with lower level of renal response at that time point (5-year RRT risk 0%, 3%, 9% and 16% for renCR, renVGPR, renPR and renNR, respectively, P<0.001, Figure 1A). Prediction of risk for RRT based on renal response depth improved at 12- and 24-months (Figure 1B-C) and at best renal response (Figure 1D). Overall survival discrimination based on renal response depth was noted as early as 12 months from therapy initiation and improved with time. Renal response criteria as best response were tested in a univariate analysis and multivariable proportional hazard models for time to RRT and OS. The graded renal response criteria demonstrated an independent prognostic role for time to RRT and OS. Along with renal stage, graded renal responses were the strongest predictors for time to RRT.
Conclusions: We validated new graded renal response criteria based on reduction in 24-h proteinuria. These 4-level renal response criteria highlight the importance of achieving a deep renal response to improve renal and overall survival. These findings will allow clinicians to make decisions on therapy changes or augmentation based on response depth as early as 6-months, before irreversible renal failure develops.
Palladini: Janssen Global Services: Honoraria, Other: advisory board fees; Siemens: Honoraria; Pfizer: Honoraria. Milani: Celgene: Other: Travel support; Janssen-Cilag: Honoraria. Schönland: Sanofi: Research Funding; Prothena: Honoraria, Other: Travel grants; Janssen: Honoraria, Other: Travel grants, Research Funding; Takeda: Honoraria, Other: Travel grants; Pfizer: Honoraria. Hegenbart: Akcea: Honoraria; Alnylam: Honoraria; Janssen: Consultancy, Research Funding; Prothena: Research Funding; Pfizer: Consultancy, Honoraria. Dispenzieri: Oncopeptides: Consultancy; Alnylam: Research Funding; Sorrento Therapeutics: Consultancy; Pfizer: Research Funding; Takeda: Research Funding; Janssen: Consultancy, Research Funding. Kumar: Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy; Carsgen: Research Funding; Beigene: Consultancy; Novartis: Research Funding; Bluebird Bio: Consultancy; Amgen: Consultancy, Research Funding; Roche-Genentech: Consultancy, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Tenebio: Research Funding; Antengene: Consultancy, Honoraria; BMS: Consultancy, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding. Kastritis: Amgen: Consultancy, Honoraria, Research Funding; Genesis Pharma: Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Pfizer: Consultancy, Honoraria, Research Funding. Dimopoulos: Takeda: Honoraria; Janssen: Honoraria; Beigene: Honoraria; BMS: Honoraria; Amgen: Honoraria. Liedtke: Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Caelum: Membership on an entity's Board of Directors or advisory committees, Other: Clinical Trial Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Alnylam: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees. Witteles: Pfizer: Honoraria, Research Funding; Alnylam: Honoraria, Research Funding; Eidos: Research Funding. Sanchorawala: Pfizer: Honoraria; Takeda: Research Funding; Celgene: Research Funding; Prothena: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees; Regeneron: Membership on an entity's Board of Directors or advisory committees; Proclara: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptide: Research Funding; Karyopharm: Research Funding; Sorrento: Research Funding; Caelum: Membership on an entity's Board of Directors or advisory committees, Research Funding. Landau: Takeda, Janssen, Caelum Biosciences, Celgene, Pfizer, Genzyme: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Genzyme: Honoraria. Cibeira: Celgene: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Akcea: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Wechalekar: Caelum Biosciences: Other: Clinical Trial Funding; Amgen: Research Funding; Janssen: Consultancy; Celgene: Honoraria; Takeda: Honoraria; Alexion, AstraZeneca Rare Disease: Consultancy. Gertz: Akcea Therapeutics, Alnylam Pharmaceuticals Inc, Prothena: Consultancy; Aurora Biopharma: Other: Stock option; Ionis Pharmaceuticals: Other: Advisory Board; AbbVie Inc, Celgene Corporation: Other: Data Safetly & Monitoring; Akcea Therapeutics, Ambry Genetics, Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Karyopharm Therapeutics, Pfizer Inc (to Institution), Sanofi Genzyme: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal